Communicating results from genetic testing
Winton Centre for Risk and Evidence Communication
Project - Communicating results from genetic testing
Patients and non-specialist healthcare professionals are increasingly being expected to understand and interpret the results of genetic or genomic testing. The reporting of these results is currently done using a wide variety of templates containing different amounts, levels and layouts of information. Guidelines recommend that genetic reports should be clear to non-specialists and patients, but there is currently little work showing exactly how that can be achieved.
We have been working with genetic specialists, non-specialist healthcare professionals, patients and members of the public to design a report template that contains the information that each needs when genetic or genomic test results are reported, and then have worked on the specific wording, numbers and graphics that might be used within that template to communicate the results and their implications clearly.
Listen to our RiskyTalk podcast episode on this issue: RiskyTalk: Communicating Genetic Risk (will download to your device)
The first piece of work, designing a template format and formulating recommendations for more user-friendly genetic reports, has been published in the European Journal of Human Genetics.
The second piece of work applied a user-centred design process to develop report templates that are specific to a particular test: cystic fibrosis (CF) carrier testing. We tested our CF report form against one currently being used in the UK, asking several hundred members of the public online questions to test how well they understood it, whether they knew what to do as a result of getting it and whether they found it easier to understand and showed that it compared very well - being rated much better on most criteria. This research has been published in Genetics in Medicine, with data and analyses available in an open source repository.
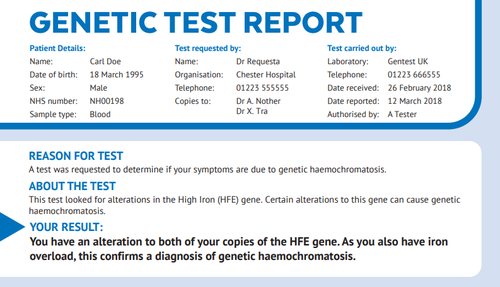
We then conducted a similar collaboration with the East Midlands and East of England Genomic Laboratory Hub on report templates for communicating the (more complex) numbers involved in having tests for pathogenic BRCA variants (associated with breast, prostate and ovarian cancers), with funding from CRUK, involving four rounds of user-centred testing with clinicians, patients, and the general public. This was followed by a summative evaluation published in May 2022 in Genetics in Medicine; key findings are summarised in this tweet thread. We have applied a similar process to develop reports for genetic haemochromatosis in collaboration with Haemochromatosis UK.
BRCA1 report templates
HFE report templates
CF report templates