Latest data from the MHRA on blood clots associated with the Astra Zeneca COVID-19 vaccine
Winton Centre for Risk and Evidence Communication
News - Latest data from the MHRA on blood clots associated with the Astra Zeneca COVID-19 vaccine
This information is from 6th May 2021 - about data up until 28th April 2021 from the MHRA in the UK.
All medical treatments have potential harms as well as potential benefits, and it's important to be able to weigh these against each other. With vaccines, the benefits are particularly complex as they can involve benefits to others as well as to ourselves - and the harms can feel particularly acute because we take vaccines when we are healthy, as a preventative measure.
With the initial release of data from the MHRA on a specific type of blood clot recorded in the UK that might be associated with the Astra-Zeneca COVID-19 vaccine, the Winton Centre were asked to help communicate the risks. Now more data is available, we have updated our graphics.
The blood-clot estimates are based on the MHRA’s yellow-card reports and so they have uncertainty around them, both because the small number of events mean there is uncertainty about the underlying risk, and that cases may yet to be reported. With very rare events like this we expect the rates to fluctuate as more data comes in so it’s not surprising to see changes from week to week.
Potential harms:
The main potential harm thought to be associated with the vaccine is a specific kind of blood clot. Cases of a severe allergic reaction (anaphylaxis) have also been recorded, but for the Astra-Zeneca vaccine, cases are very rare, perhaps because of precautions being taken for those with known allergies. Other side effects are so far thought to be short term.
The MHRA data point to these specific blood clots being more common in younger people.
Potential benefits:
The benefits of the vaccine are protection against COVID-19 (short-term and 'long COVID') - for the person being vaccinated and for those they come into contact with as it also reduces the chances of them being infected by the vaccinated person.
These potential benefits, though, change according to:
The potential benefits also accrue every day that the person is vaccinated (and exposed to the virus).
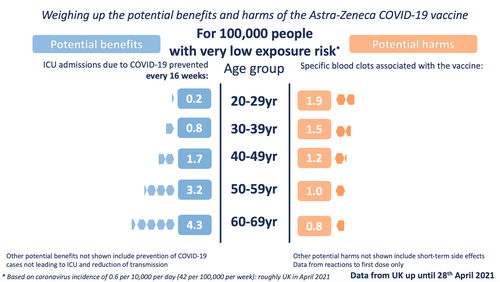
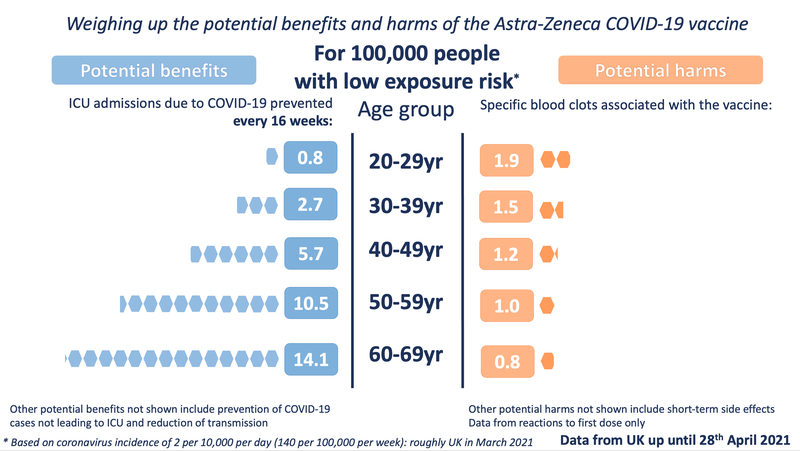
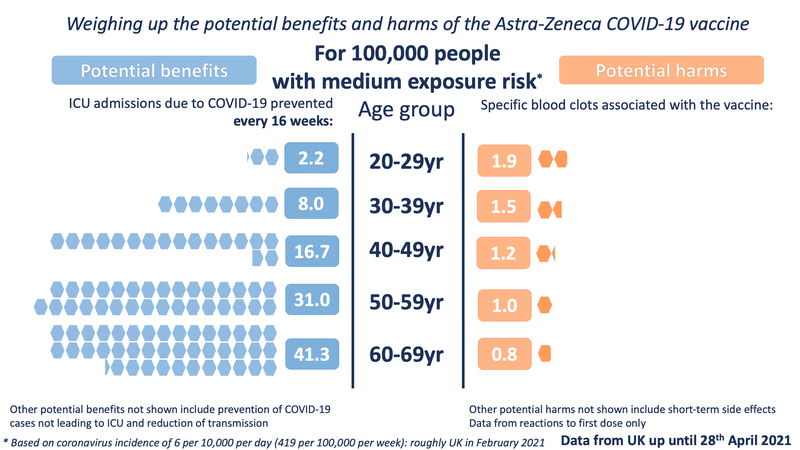
In order to illustrate the potential benefits weighed up against the potential harms, we wanted to choose 'benefits' that might be considered comparable to the most serious harms - those of the specific blood clots being discussed. We chose to illustrate the estimated number of ICU hospitalisations prevented by the vaccine (per age band). We also chose to illustrate three possible levels of exposure to the virus - each time illustrating the benefits accrued over 16 weeks.
In the latest graphics we have included a mention of the other potential benefits and harms on the graphic itself to ensure that these are always made explicit. We also changed the icon from the circles we used in the previous graphics to try to avoid confusion between the two different datasets.
Data used:
For the potential benefit: incidence rates based on the Covid-19 Infection Survey, ONS, 30 April 2021. The proportion of hospitalisations in a cohort was calculated using the estimates of COVID-19 hospitalisation rates associated with the 10-year age cohorts studied. These estimates were taken from Table 1 of the 29 July 2020 report of the Scientific Pandemic Influenza Group on Modelling, Operational sub-group (SPI-M-O). The proportion of ICU cases to hospitalisations was calculated using the PHE Benefit Estimation for COVID-19 Report from 3 April 2021 which gave more recent ICU figures than the July 2020 report - the proportion of COVID-19 patients going into ICU has fallen as treatments have improved. The 10-year age cohorts were determined by weighted averages if not directly available. A fixed vaccine efficacy of 80% for all age groups for ICU reduction was used.
For the potential harms: numbers of cases of the blood clot reactions provided by MHRA up until April 28th in five-year age-bands (the data has not yet been publicly released in 5-year age bands). These observed rates were smoothed using a Poisson regression on age, with log-link.
The 'very low exposure' image shows the benefits calculated with an incidence level of 0.6 (roughly the current - as of 30th April - incidence level of COVID-19 in the UK). Of course it's important to note that vaccination benefits continue to accrue over time and although we're at low incidence now, it may increase again in the future - and even if it remains this low, the vaccination should give benefits over longer than 16 weeks.
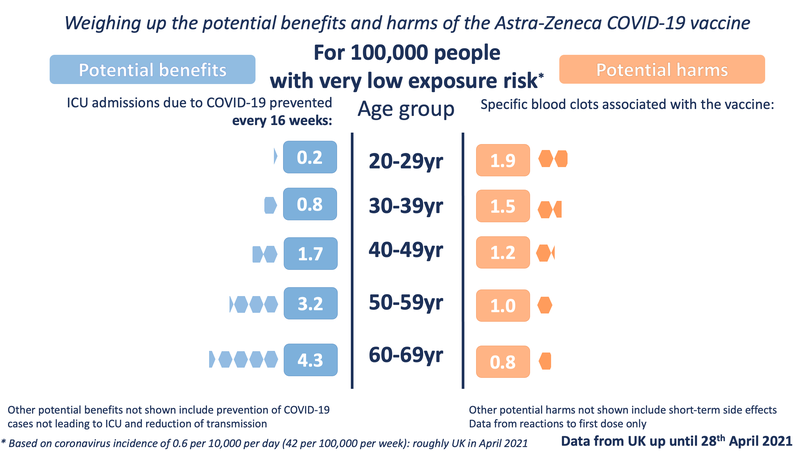
The other three exposure levels are the same as in our previous images:
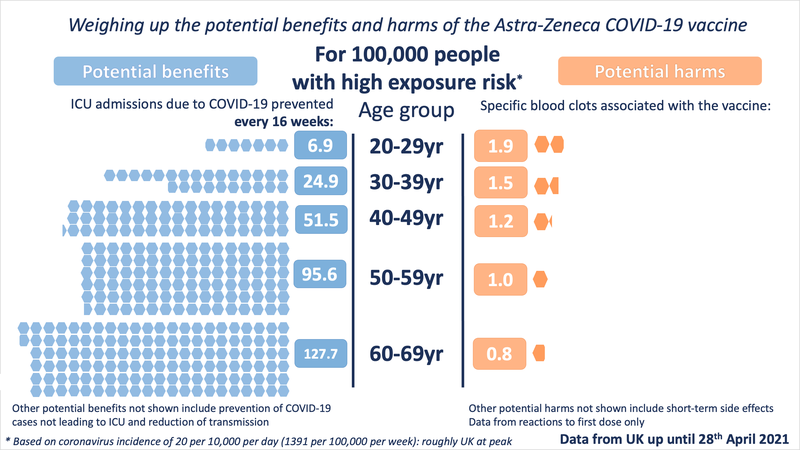
(These images are CC-BY-NC. Please credit "Winton Centre for Risk & Evidence Communication, University of Cambridge")
These illustrations show the approximate balance as it would be for people of different ages, over 16 weeks, at four different exposures to the virus (which would depend on local prevalence of the virus and how much an individual was exposed to other people who might be carrying it). People with underlying health conditions that increase their risk of a poor outcome from COVID-19 would have a higher benefit from the vaccine than illustrated for their age-range. People without underlying health conditions would have a slightly lower benefit than illustrated.
We have also looked at the data broken down by sex. Currently the MHRA data shows no statistically significant difference between the likelihood of men or women developing the specific blood clots - although there are slightly more cases in women than men, this could be due to chance variation, or possibly under-reporting amongst men. As more data comes in, this potential difference will be closely monitored. By contrast, the potential benefits to men and women, particularly over the age of around 40, do differ, with men receiving a greater potential benefit because they are more at risk of a poor outcome from COVID-19 :
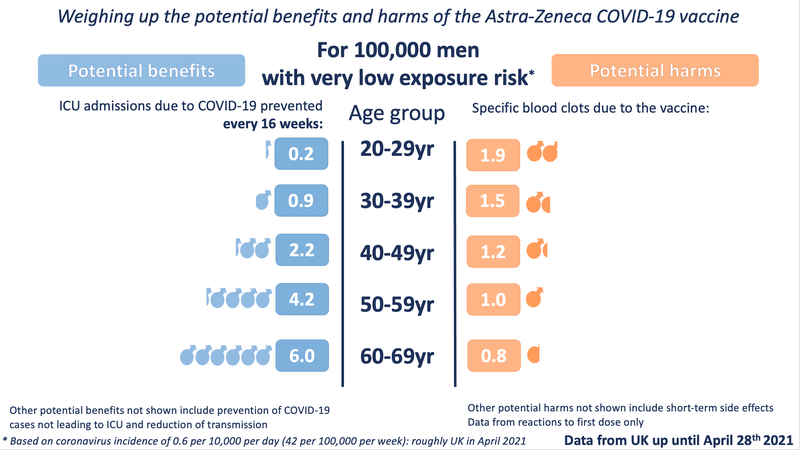
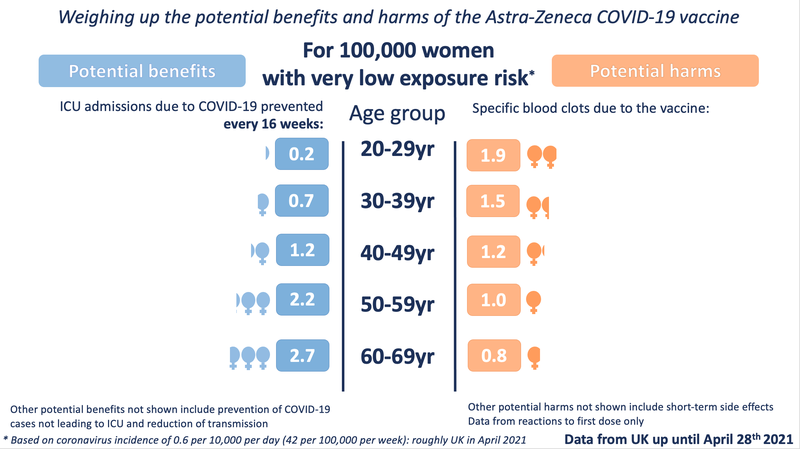
( Male / Female ICU admission differences were calculated using the Age and Sex Distribution Data from ICNARC, Covid-19 Report, 26 March 2021 )
It is very important to note that the benefits shown are approximate, as taken at a constant level of exposure to the virus over 16 weeks (very few in the UK would be likely to experience 16 weeks at the highest exposure rate). A vaccinated person will keep accruing this benefit over the lifetime of the vaccine’s protection. The risk from vaccination occurs only at the point of vaccination. This means that over time, the benefits will increase but the risks will not.
It is also important to note that the benefits illustrated are only for ICU admission due to COVID-19. For every 1 person shown as being saved from ICU admission, there are many more who might be being saved from suffering hospitalization and ‘long COVID’. We are also not illustrating the benefit of not spreading the virus to others.
When making decisions, it is also important to take into account other potential vaccines available. For example, if there were an equally effective vaccine available, immediately, that did not carry the risk of a blood clot reaction then that might swing a decision in favour of taking that vaccine in preference to the Astra-Zeneca vaccine. However, if such a vaccine were not immediately available, then the risk of exposure to the COVID-19 virus during each week of any delay before such an alternative were available would have to be weighed up in a decision whether to wait or not.
All of these factors make any decision over the Astra-Zeneca vaccine a complex one - the risk:benefit ratio varies between different people, and as prevalence of the virus changes. We hope these illustrations help make these complexities slightly clearer.
European Medicines Agency information: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood
Medicines and Healthcare products Regulations Agency information: https://www.gov.uk/government/news/mhra-issues-new-advice-concluding-a-possible-link-between-covid-19-vaccine-astrazeneca-and-extremely-rare-unlikely-to-occur-blood-clots